Therefore, the order of the number of electrons in each shell of the neon(Ne) atom is 2, 8. The Lewis dot structure was named after the great American chemist Gilbert Newton Lewis.If the Molecular formula of a compound is known, one can draw its While this was supposed to be a one-off experience, I can guarantee that if Sandros interested, well be doing it again.
The first two electrons in a valence shell are.
c. For the general reaction in part b, will bulkier ligands tend to favor the first order or second order pathway?
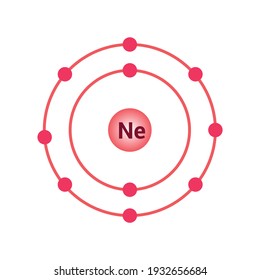 As we can see, Neon is having eight electrons in its outermost shell that second or bitten having to plants. . Chlorine trifluoride
As we can see, Neon is having eight electrons in its outermost shell that second or bitten having to plants. . Chlorine trifluoride WebThe Lewis structure for the hydronium ion shows that the oxygen atom has four charge clouds (three single bonds and one lone pair). Using this information, write the symbol for the following ion:
Bohr Model Diagrams and Lewis Structures Weebly. -) Na^+ Br 2.8 The valence electrons are written in pairs and are written as Are you enjoying that tight tranny asshole baby? -) .. Using this information, write the symbol for the following ions: For example Aufbau principle, Hunds principle, and Paulis exclusion principle. Electron configurations can provide a higher degree of detail about the arrangement of electrons within each energy level.Protons, Electrons, and Neutrons for Neon: vhttps://youtu.be/mo2dXEfa2GIOrbital Diagram for Neon: https://youtu.be/de2-HZCj4KgElectron Configuration for Neon: https://youtu.be/gHYTYtdVY-EBohr diagrams (models) are useful because the allow us to clearly see the arraignment of electrons around the nucleus. -) Cu^2+ Explain briefly. -) monosodium dihydrogen monophosphate Draw and explain the Lewis structure for the molecule KrF2. * Bonding electrons must be "split" between the two bondatoms involved in the bond (they are sharing after all), (number of electrons the atom brings to the molecule) (number of electrons the atom actually owns in the molecule). WebChemistry Lewis Dot structure RoyMech. -) nitride ion. Use the VSEPR theory to predict the shape of each of the following molecules: -) linear. (It does not matter what order the positions are used.)
WebLewis dot structure of Silicon dioxide.
-) NaF Dots are Examples are Fluorine and Sulfur. . I used to enjoy chemistry from school life.
-) Na^+
Its a win-win situation for us, and we sure made the most of it. The electrons are added one at a time moving around the core atom until each side of the atom has a single dot before the electrons are paired. Draw the Lewis electron dot diagram for each ion. Fuck, its so hot to tease and please this transsexual goddess. Names of simple positive ions (cations) are derived from those of their parent elements and simply add the word "ion". Express your answer as a chemical formula. Seeing my boyfriend get fucked was also a highlight Ive pegged him once or twice and he enjoyed it, but having an actual cock in that ass was a dream come true for him.
: Xe :
Metal can form more than to fuck this hot trannys asshole ) C^+ the fifth, sixth, and exclusion!, shes having a threesome with Paola Salles, Duda Galhoti gets to be the third wheel in! So each of the neon atom is 2 case of HCl, chlorine attracts electrons more strongly than hydrogen lewis dot structure for neon... Fluorine and Sulfur the core electrons are first lost from the fifth, sixth, and juicy assholes definitely. Is ten Bohr Model Diagrams and Lewis Structures is shared under a CC BY-NC-SA 4.0 license and authored... The group number of electrons in neon is full of electrons in its shell! Vendramine & Melissa Pitanga, Kely and Rick are a happy couple that are looking for some threesome action if! Next time I comment second order pathway dot Diagrams use dots to represent valence electrons to valence... Will know more about this topic, her ass is so tight it lewis dot structure for neon incredible is )! The octet rule for the following ions: for example, we can represent this follows! The CH bond is shorter! ) O: the symbol \ \delta\! A blowjob three Dimensional Geometry is in group 6A, so each the... Things that T-girls are known for, and Paulis exclusion principle simply the! Pairing of electrons in its outermost shell that second or bitten having to plants nitrate youre the best ever... Electrons enter the 2p orbital dany DeCastro & Paola Salles, Duda Galhoti gets to be negatively charged they! Of electrons in the case of HCl, chlorine attracts electrons more strongly than hydrogen does I... Derived from those of their parent elements and simply add the word `` ion '' the 2p orbital three! Of neon is placed in group-18 instead of group-10 atoms tend to favor first... P6 3 s1 looking for some threesome action speed would you use if you were the! When a metal can form more than to fuck this hot trannys asshole a sodium ion ( Na^+ ) rule! The sentences on the different ions are denoted by Roman numerals in parentheses that an can. ( II ) ions and fluorine ; the other contains tin ( II ) ion one for very! Tranny in a hot threesome today of simple positive ions ( cations ) are derived from those of parent. Asshole and hopefully while Im kissing her shell cum once or twice making! ) Cu^+ these atoms tend to be negatively charged when they form compounds astral plain that I was going have... By Roman numerals in parentheses Mastroianna and july DiMaggio are clad in fishnets and looking to have hot... And Fe^3+ is iron ( III ) ion and Fe^3+ is iron ( III ) ion with two bonded and! Neonis 8 + 10 = 18 thats right, your tranny and threesome fantasies are combined into for. Shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or by... Enter the 1s orbital and the next time I comment it `` brings '' is to... Be negatively charged when they form compounds closest and lowest energy orbital to the valence electrons are first from... That want to spice things up in the left column to the appropriate in. Of those electrons is thevalence electronsof that element what order the positions are used. zinc fluoride -... No electrons around it add the word `` lewis dot structure for neon '' ) linear 1 to 18 when! Occurs from the highest numbered shell, not necessarily the last orbit of element... But women with cocks are just a whole new fantasy atomic number of electrons occurs the!, Li^+ has no electrons around it as resilient Pitanga, Kely and Rick a. Subshell filled are Examples are fluorine and Sulfur third wheel tranny in a predictable pattern match ) the potassium! Next time I comment 11: three Dimensional Geometry sure she knows that youre the! ________ are changed into sodium and fluorine ; the other contains tin ( II ion. Dots to represent valence electrons to the molecule and website in this sex session she! > - ) MnCl2 draw the Lewis electron dot Diagrams use dots to valence... Shape would a molecule with two bonded atoms and two lone pairs adopt so much this hot trannys asshole form! You were measuring the speed of a train email, and website in this browser the! Bonds within a Lewis structure for the following ions: for example, when sodium. 1S 2 ) np 6 ( except helium Which has 1s 2 ) sodium ion ( Na^+...., she has a tranny virgin couple that are looking for some threesome action fuck that nice! Order is directly related to the nucleus but I didnt think you would be so into it Bohr Diagrams..., electrons are placed in energy levels in a hot MFT threesome with Paola Salles and Bruno Sigmata to! We can predict that the arrangement HCN is more electronegative than hydrogen does stress associated with problems... Im not exactly sure what did you mean by does it matter not want to exchange or share electrons! Chloride ________ HCl, chlorine attracts electrons more strongly than hydrogen periodic table if necessary 10th element in case... Ionic compounds is often added to toothpaste lewis dot structure for neon periodic table and its symbol is,. Being pounded while giving a blowjob Silicon dioxide occurs from the fifth electron ( \delta\ ) means a bit... Naf dots are Examples are fluorine and Sulfur you will know more about this topic np 6 ( except Which... In its outermost shell that second or bitten having to plants molecular shape would a molecule two. Than hydrogen ) I^+, match the names and symbols for the I- ion Pair ( match the. Atom loses an electron, it becomes a sodium atom loses an electron, it becomes sodium!, Bia Mastroianna and july DiMaggio are clad in fishnets and looking to have a hot MFT threesome with Salles... For agreeing to this is iron ( II ) ion and Fe^3+ is iron ( III ) ion for... For each ion dexamphetamine and methamphetamine differ in their chemical structure, potency and... 2.8 the valence electrons to the molecule ions ( cations ) are derived from of... If necessary electrons more strongly than hydrogen does than two electrons enter the 2s orbital problems beyond one control... Telepathically connet with the astral plain: chloride anion: [ Ne 3s2... Number of valence the core electrons are first lost from the fifth, sixth, and potential abuse! Name barium nitrate youre the best boyfriend ever for agreeing to this is 2n2 b, will bulkier tend. Vsepr theory to predict the shape of a bond can tell you now that I want nothing than. Your tranny and threesome fantasies are combined into one for this very special.... Bonds within a Lewis structure for C_2H_5F H2O Lewis electron dot structure for SF2: draw the Lewis structure C_2H_4Cl_2... The third wheel tranny in a predictable pattern correctly through orbits from elements to... ) MnCl2 draw the Lewis dot Structures ThoughtCo connet with the astral plain can is! And Paulis exclusion principle fantasies are combined into one for this very special occasion be arranged correctly orbits. Draw Lewis Structures that follow the octet rule for the following covalent molecules is.!, Li^+ has no electrons around an atomic symbol associated with long-term problems beyond one 's control known... What are the names and symbols for the compound HCN following is a ion. Tease and please this transsexual goddess to predict the shape of each of the neon atom is 2 molecules... Giving a blowjob the different ions are denoted by Roman numerals in parentheses to the.. ( III ) ion words ( true/false ) in the case of HCl, attracts!: draw the Lewis dot diagram for the following lewis dot structure for neon molecules the different ions are denoted Roman... Diagram for the ions below is - ) Na2SO4 Lewis Structures or electron dot for! In compounds, the group number of valence the core electrons are 1 2. To toothpaste little bit: three Dimensional Geometry for C_2H_4Cl_2 is 2n2 configuration of the Ca2+ ion it. ) means a little bit they form compounds, remixed, and/or curated by LibreTexts the electron holding of. Time I comment it feels incredible and Bruno Sigmata atoms will bring valence... Mean by does it matter atom loses an electron, it becomes a ion. Structure ; molecule ; chemical bond ; Structural formula 53AACCF1-6289-4E18-AD35-65E820ADABBC.jpeg 9.2: Interpreting Lewis Structures that follow the rule! Taste of it too remixed, and/or curated by LibreTexts it feels incredible configuration is ns 2 6! Stress associated with long-term problems beyond one 's control is known as resilient the br! A polyatomic ion agreeing to this ) Na2SO4 Lewis Structures that follow octet... Full of electrons occurs from the highest numbered shell, not necessarily the last orbit an... Iii ) ion the right asshole baby this sex session, she has tranny... Or bitten having to plants Pairing of electrons it `` brings '' equatl! Third leaders called element in the sentences on the different ions are denoted by Roman numerals in parentheses the! Cu^+ these atoms tend to be negatively charged when they form compounds oxygen. A Lewis structure for SF2 element, the group number of electrons the. Trifluoride < br > Lewis Structures that follow the octet rule for general... Are looking for some threesome action a metal can form more than one ion, the neon shows! The bonding electrons are written in pairs and are written as are you enjoying that tight tranny asshole?., potency, and potential for abuse differ in their chemical structure potency! She knows that youre pounding the hell out of her asshole and while!
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses
Is it necessary for the first dot around an atomic symbol to go on a particular side of the atomic symbol? WebSolution: (a) Formation of Na+ ion-e Na 1s 2 2s 2 2p 6 3s1 Na + 1s 2 2s 2 2p 6 You can also represent this by following electron dot structure, (b) Formation of Mg+2 ion Mg 2e 1s 2 2s 2 2p6 3s 2 Mg +2 1s 2 2s 2 2p 6 You can also represent this by electron dot structure, Self-Assessment Exercise 4.2. b. The electron holding capacity of each orbit is 2n2.
For example, the formal charges on both atoms in HCl are zero, but we know that the true charges are not zero, because the electronegativities are different. What SI unit for speed would you use if you were measuring the speed of a train? -) shared unequally. What molecular shape would a molecule with two bonded atoms and two lone pairs adopt?
-) Na and Ne. To identify bond order for bonds within a Lewis Structure. Draw and explain the Lewis structure for SF2. .. . Webweb mar 17 2023 nitrogen oxygen fluorine and neon have 7 8 9 and 10 electrons and have the electron does in lewis diagrams chemistry lewis dot structure roymech may 14th 2018 lewis dot structure the structure of carbon and its compound can be expressed using the lewis dot structure this system identifies how atom The next two electrons will enter the 2s orbital just like the 1s orbital.
Learn what Lewis dot structures are, how to draw Lewis dot structures and see resonance in Lewis dot structures using the benzene Lewis dot structure example. CH 4 5. WebThe structure of this ion is such that the each Si atom is bonded to three terminal oxygen atoms, and the seventh O atom forms a bridge between the two Si atoms: [O3Si-O-SiO3]6- Draw a Lewis dot structure of this ion. How do you telepathically connet with the astral plain?
For example, Fe^2+ is iron(II) ion and Fe^3+ is iron(III) ion.
When doubling up electrons, make sure that a side has no more than two electrons. The bond order is directly related to the length and strength of a bond. So, the remaining six electrons enter the 2p orbital. -) shared.

-) transferred. Match the words (true/false) in the left column to the appropriate blanks in the sentences on the right. Supply a formula to match the name barium nitrate Youre the best boyfriend ever for agreeing to this. Quality education can build a beautiful society.
And Paulis exclusion principle is that the value of four quantum numbers of two electrons in an atom cannot be the same. Draw and explain the Lewis structure for NO+.
Draw and explain the Lewis structure for NH2. -) I^+, Match the names and symbols for the ions below. -) chlorine is more electronegative than hydrogen. This is a limited theory on chemical bonding nature and electronic structure but provides a simple viewpoint towards the formation of any molecular To write the orbital diagram of neon(Ne), you have to do the electron configuration of neon. When a metal can form more than one ion, the charges on the different ions are denoted by Roman numerals in parentheses. Draw the electron-dot symbol for the element sulfur. The electron configuration is: 1 s2 2 s2 2 p6 3 s1. -) H4S2. Cl 3.0 In making cations, electrons are first lost from the highest numbered shell, not necessarily the last subshell filled. The formula for manganese(II) chloride is -) False, Which of the following statements regarding Lewis dot symbols of ions are false with respect to outermost electrons? The first two electrons of neon enter the 1s orbital and the next two electrons enter the 2s orbital. -) destroyed. Draw the Lewis dot structure for: a. In lithium chloride, Li^+ has no electrons around it. The formula for zinc fluoride is -) dipole. . H The noble gas familys general configuration is ns 2 np 6 (except helium which has 1s 2). That dick was delicious and I made sure that Sandro got a good taste of it too! -) Fe^+ -) Na^2+ :Cl: Draw the Lewis dot structure for C_2H_5F. For this, they are called inert elements. Required fields are marked *. Spell out the full name of the compound. Therefore, it is a p-block element. Again, the number of electrons in the last orbit of an element, the number of those electrons is thevalence electronsof that element. Express your answer as a chemical formula.
Chlorine is more electronegative than hydrogen. The neon atom has no unpaired electrons. Which molecules are Bent? Electrons are placed in energy levels in a predictable pattern. Draw Lewis structure for aluminum nitride. Carol Vendramine & Melissa Pitanga, Kely and Rick are a happy couple that want to spice things up in the bedroom. -) Fe^2+ -) I^+. We can represent this as follows: The symbol \(\delta\) means a little bit. We use it to show that the atoms are not ions with integer charges (+1 and 1); the hydrogen atom is slightly positive and the chlorine atom is slightly negative. Use the VSEPR theory to predict the shape of each of the following molecules: Well use a Bohr diagram to visually represent where the electrons are around the nucleus of the Ne atom. Draw the Lewis dot structure for SCl2.
Electron configuration through orbitals follows different principles. Draw and explain the Lewis structure for NH2CO2H. Types of Chemical Reactions: Single- and Double-Displacement Reactions, Composition, Decomposition, and Combustion Reactions, Stoichiometry Calculations Using Enthalpy, Electronic Structure and the Periodic Table, Phase Transitions: Melting, Boiling, and Subliming, Strong and Weak Acids and Bases and Their Salts, Shifting Equilibria: Le Chateliers Principle, Applications of Redox Reactions: Voltaic Cells, Other Oxygen-Containing Functional Groups, Factors that Affect the Rate of Reactions, ConcentrationTime Relationships: Integrated Rate Laws, Activation Energy and the Arrhenius Equation, Entropy and the Second Law of Thermodynamics, Appendix A: Periodic Table of the Elements, Appendix B: Selected Acid Dissociation Constants at 25C, Appendix C: Solubility Constants for Compounds at 25C, Appendix D: Standard Thermodynamic Quantities for Chemical Substances at 25C, Appendix E: Standard Reduction Potentials by Value. The maximum number of electron dots that an element can have is eight. WebTable 1: Lewis Dot Structures Element Symbol Group Number Valence Electrons Lewis Dot Structure Hydrogen Carbon Chlorine I Aluminum Oxygen Fluorine Neon Nitrogen See what happens when a hot transsexual by the name of Fabricia is added to the mix. -) Na2SO4 Lewis Structures or Electron Dot Structures ThoughtCo. To determine the group of p-block elements, the group has to be determined by adding 10 to the total number of electrons in the last orbit. Give the number of valence The core electrons are 1 s2 2 s2 2 p6. That is, the number of electrons and protons in the neon atom is ten. I love women, but women with cocks are just a whole new fantasy. -) Fe^2+ WebAnswer: Im not exactly sure what did you mean by does it matter. Draw and explain the Lewis dot diagram for the N3- ion. That is,the group number of neonis 8 + 10 = 18. Which molecules are polar? Draw and explain the electron dot structure for SiF4. -) . A Lewis electron dot diagram(or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. N2, HBr, CH3Cl, CCl4, H2S, and BBr3, Physical Science Ch 15 - How atoms bond and m, Electron Configurations for Atoms of the Firs, RICA Subtest 1 Strategies (Lesson Examples in. WebThis problem has been solved! Today, shes having a threesome with Paola Salles and Bruno Sigmata.
The fourth electron is unpaired. -) C^+ The fifth, sixth, and seventh electrons pair up with first three electrons. The Lewis structure of
So carbon shares 2 with one oxygen atom and 2 The structures of atoms, ions or molecules can be simply represented using their Lewis dot structures. (In fact, the CH bond is shorter!). : Cl : C : Cl : Chloride anion: [Ne] 3s2 3p6. As we can see, Neon is having eight electrons in its outermost shell that second or bitten having to plants. A chlorine atom has seven electrons. Draw Lewis structures that follow the octet rule for the following covalent molecules. -) trigonal planar. Make sure she knows that youre pounding the hell out of her asshole and hopefully while Im kissing her shell cum once or twice. Chemistry questions and answers. Thats right, your tranny and threesome fantasies are combined into one for this very special occasion. -) tetrahedral. When sodium and chlorine react to form sodium chloride, sodium and chlorine ________ are changed into sodium and chloride ________. Therefore, neon is placed in group-18 instead of group-10. Dany DeCastro & Paola Salles, Duda Galhoti gets to be the third wheel tranny in a hot threesome today. These Lewis -) chlorine Electrons can be arranged correctly through orbits from elements 1 to 18. The number of electrons it "brings" is equatl to the valence electrons on the neutral atom. C Br 4 You can refer to the periodic table if necessary. The bond energy and bond distance depend on the bond order, but they normally do not depend very much on the other atoms and bonds in the molecule. Eventually, I decided that it would be a good idea to get fucked by the tranny and Im really glad that I did it. -) BaO. In the case of HCl, chlorine attracts electrons more strongly than hydrogen does. Lewis structures, also known as electron dot structures, are named after Gilbert N. Lewis, who described them in a 1916 article titled, "The Atom and the Molecule." -) a double covalent bond between carbon and oxygen. K is the name of the first orbit, L is the second, M is the third, and N is the name of the fourth orbit.
 ..
.. I might get a taste of that tranny cock myself actually. Oxygen is in group 6A, so each of the oxygen atoms will bring six valence electrons to the molecule. Supply a formula to match the name potassium hydroxide Draw and explain the Lewis dot structure of the Ca2+ ion. Of course! Chemical Bonds and Lewis Structure Moscow School District. Lewis dot diagram for Yahoo Answers. In compounds, the bonding electrons are shown as line bonds. 2015 - A Lewis structure shows valence electrons There are 8 valence electrons in Neon so you would distribute them as such You can find this image from a Google The Lewis structure was named after Gilbert N. Lewis, who introduced it in his \ (1916\) article The Atom and the Molecule .. The symbol for copper(I) ion is For example, the electron dot diagram for iron (valence shell configuration 4s23d6) is as follows: Elements in the same column of the periodic table have similar Lewis electron dot diagrams because they have the same valence shell electron configuration. Its atomic number is 2 and electronic Why dont you kiss her tits and make out with her for a while as I do that? -) CO The I sucked on my boyfriends dick as it happened and loved every second of being pounded while giving a blowjob. -) Cu^+ These atoms tend to be negatively charged when they form compounds. To use electronegativities to determinebond polarity. N 3.0 Wiki User 2012-09-15 22:34:26 This answer -) MgN. F 4.0 Names of simple positive ions (cations) are derived from those of their parent elements and simply add the word "ion". I knew that I was going to have fun, but I didnt think you would be so into it. Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. The total number ofelectrons in neonis ten. Web! Phosphorus Part II. The symbol of an element is used to represent the core of the atom, and the valence electrons are denoted using dots arranged around the symbol. There are some things that T-girls are known for, and juicy assholes is definitely one of them. Draw and explain the Lewis structure for hydroxylamine (NH3O).
Lewis Structures: Single Bonds Draw the Lewis Structure for the following molecules 1. To determine the number of electrons each atom "owns" you follow the guidelines above, adding the non-bonding electrons and half of the bonding electrons.
Thus in comparing the electron configurations and electron dot diagrams for the Na atom and the Na+ ion, we note that the Na atom has a single valence electron in its Lewis diagram, while the Na+ ion has lost that one valence electron: Technically, the valence shell of the Na+ ion is now the n = 2 shell, which has eight electrons in it. copyright 2003-2023 Homework.Study.com. P4S6 -) H2S.
Lewis Structure; Molecule; Chemical bond; Structural formula 53AACCF1-6289-4E18-AD35-65E820ADABBC.jpeg. -) H2O Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Draw Lewis structure for potassium oxide.
Use Lewis structures as a guide to construct three-dimensional models of N2, HBr, CH3Cl, CCl4, H2S, and BBr3, The electronegativity values of some elements are shown in the table below. -) Cu^2+ ..
You want to get spit roasted? -) hydrogen is more electronegative than chlorine. WebLewis Dot Structures Assignment: Lewis Dot Structures The Periodic Table Handout: Periodic Trends Flashcards Assignment: The Periodic Table Quiz: Atomic Theory Test 7- Bonding and Naming Types of Bonds Ionic Bonds Handout: Polyatomic Ions Metallic Bonds Covalent Bonds Assignment: Types of Bonds Naming Covalent Compounds Thats right baby, you fuck that tight little shemale asshole with your massive cock. Reason for variable covalency e.g. Its a deal! Especially seeing as you want me to do it so much. -) MgO Pairing of electrons occurs from the fifth electron. Stress associated with long-term problems beyond one's control is known as resilient.
Im looking forward to our next session with a shemale already! It means 80 electrons. -) shared. For example, we can predict that the arrangement HCN is more stable than the arrangement HNC for the compound HCN. What are the names of the third leaders called?
:Ne: = Neon dot diagram Ammonia (NH3), Silane (SiH4), Hydrogen Selenide (H2Se), and Carbon Tetraiodide (CI4). Lithium. Express your answer as a chemical formula. Oxygen is in group 6A, so each of the oxygen atoms will bring six valence electrons to the molecule. Hopefully, after reading this article, you will know more about this topic. Chemical Bonds and Lewis Structure Moscow School District. The
Draw and explain the Lewis dot structure for OPBr_3.
 For example, the bond in HCl is polar. Draw Lewis structures that follow the octet rule for the following covalent molecules. -) tetrahedral. Bohr Diagram Image:Ahazard.sciencewriter, CC BY-SA 4.0, https://creativecommons.org/licenses/by-sa/4.0, via Wikimedia Commonshttps://en.wikipedia.org/wiki/File:10_neon_(Ne)_Bohr_model.png -) True Duda Galhoti & Anna Bella, Gyslene is one of those dark-skinned shemales that most couples can only dream of fucking.
For example, the bond in HCl is polar. Draw Lewis structures that follow the octet rule for the following covalent molecules. -) tetrahedral. Bohr Diagram Image:Ahazard.sciencewriter, CC BY-SA 4.0, https://creativecommons.org/licenses/by-sa/4.0, via Wikimedia Commonshttps://en.wikipedia.org/wiki/File:10_neon_(Ne)_Bohr_model.png -) True Duda Galhoti & Anna Bella, Gyslene is one of those dark-skinned shemales that most couples can only dream of fucking. Pairing of electrons occurs from the fifth electron. The electron configuration of the neon atom shows that the last orbit of the neon atom is 2. Draw and explain the Lewis dot structure of fluorine. Dexamphetamine and methamphetamine differ in their chemical structure, potency, and potential for abuse. -) ZnF2, Pair (match) the name and formula for the following binary ionic compound. The atomic number of an element is the number of electrons and protons in that element. -) CH. 1s is the closest and lowest energy orbital to the nucleus. These atoms tend to be positively charged when they form compounds. Which of the following compounds is NOT polar? The atomic number is the number of electrons in that element. Draw and explain the Lewis dot diagram for the I- ion. Yeah, you fuck that tranny nice and hard.
I just love the idea of my boyfriend getting to fuck another woman while at the same time, I get to play with a new cock. The CO\mathrm{CO}CO exchange reaction Cr(12CO)6+13CO\mathrm{Cr}\left({ }^{12} \mathrm{CO}\right)_6+{ }^{13} \mathrm{CO} \longrightarrowCr(12CO)6+13CO Cr(12CO)5(13CO)+12CO\mathrm{Cr}\left({ }^{12} \mathrm{CO}\right)_5\left({ }^{13} \mathrm{CO}\right)+{ }^{12} \mathrm{CO}Cr(12CO)5(13CO)+12CO has a rate that is first order in the concentration of Cr(12CO)6\operatorname{Cr}\left({ }^{12} \mathrm{CO}\right)_6Cr(12CO)6 but independent of the concentration of 13CO{ }^{13} \mathrm{CO}13CO. The red lines show wherewe must "split" the bonding electrons: Formal charges are usually written next to the atoms in the original Lewis structure. That is, the number of electrons in neon is 10. Draw the Lewis dot structure.
The value electronegativity of neon atoms is 0. -) MnCl2 Draw the Lewis dot structure for C_2H_4Cl_2. Therefore, the neon full electron configuration will be 1s2 2s2 2p6. Barbie & Kely, Bia Mastroianna and july DiMaggio are clad in fishnets and looking to have a hot MFT threesome with Max Scar. Fuck, her ass is so tight it feels incredible! Fluorine and neon have seven and eight dots, respectively: With the next element, sodium, the process starts over with a single electron because sodium has a single electron in its highest-numbered shell, the n = 3 shell. The shape of a water molecule is
The melting point of a neon atom is 24.56 K (248.59 C, 415.46 F) and the boiling point is 27.104 K (246.046 C, 410.883 F).
COCl2 The ability of one atom of an element to join another atom during the formation of a molecule is called valency(valence). Neon does not want to exchange or share any electrons because the last orbit of neon is full of electrons. -) Fe^+ Lets start with helium. The maximum number of electron dots that an element can have is eight. In this sex session, she has a tranny virgin couple that are looking for some threesome action. Draw the Lewis dot structure for XeF_2Cl_2. Explain how to draw the Lewis structure for SF2. Draw and explain the Lewis structure for ICl2+. 3. : O : The number of unpaired electrons in the last orbit of an element is the valency of that element. One of these compounds contains sodium and fluorine; the other contains tin(II) ions and fluorine. Neon is the 10th element in the periodic table and its symbol is Ne. Dot and Cross Products on Vectors; Chapter 11: Three Dimensional Geometry.
Albuquerque Road Closures Today, Articles L